Thursday, January 01, 2009
HAPPY NEW YEAR!!!!!!!!!!!
I want to wish everyone a Happy New Year!!!!!!!!!!!!
Thanks to all of the readers of this blog for your support and contributions during 2008.........unfortunately I must put this blog on hiatus once again............
I have been unemployed for the last 2 months ( which is why you may have noticed an increase in postings).........However I have been offered and accepted a job in Eastern Washington and I don't know if I'll have internet access over there........I will get back to this blog whenever I can and continue to track what the "crazies" in D.C. are up to.........We have hope for change in the new year and it will truly begin on Jan.20th........the extent of change is still yet to be determined.......So I hope and pray for a better world in 2009.............
GOOD LUCK to us all !!!!!!!!!!!............... PEACE............... Scott
January 1:
1959 : Batista forced out by Castro-led revolution On this day in 1959, facing a popular revolution spearheaded by Fidel Castro's 26th of July Movement, Cuban dictator Fulgencio Batista flees the island nation. Amid celebration and chaos in the Cuban capitol of Havana, the U.S. debated how best to deal with the radical Castro and the ominous rumblings of anti-Americanism in Cuba. The U.S. government had supported Batista, a former soldier and Cuban dictator from 1933 to 1944, who seized power for a second time in a 1952 coup. After Castro and a group of followers, including the South American revolutionary Che Guevara (1928-1967), landed in Cuba to unseat the dictator in December 1956, the U.S. continued to back Batista. Suspicious of what they believed to be Castro's leftist ideology and worried that his ultimate goals might include attacks on the U.S.'s significant investments and property in Cuba, American officials were nearly unanimous in opposing his revolutionary movement. Cuban support for Castro's revolution, however, grew in the late 1950s, partially due to his charisma and nationalistic rhetoric, but also because of increasingly rampant corruption, greed, brutality and inefficiency within the Batista government. This reality forced the U.S. to slowly withdraw its support from Batista and begin a search in Cuba for an alternative to both the dictator and Castro; these efforts failed. On January 1, 1959, Batista and a number of his supporters fled Cuba for the Dominican Republic. Tens of thousands of Cubans (and thousands of Cuban Americans in the U.S.) celebrated the end of the dictator's regime. Castro's supporters moved quickly to establish their power. Judge Manuel Urrutia was named as provisional president. Castro and his band of guerrilla fighters triumphantly entered Havana on January 7. The U.S. attitude toward the new revolutionary government soon changed from cautiously suspicious to downright hostile. After Castro nationalized American-owned property, allied himself with the Communist Party and grew friendlier with the Soviet Union, America's Cold War enemy, the U.S severed diplomatic and economic ties with Cuba and enacted a trade and travel embargo that remains in effect today. In April 1961, the U.S. launched the Bay of Pigs invasion, an unsuccessful attempt to remove Castro from power. Subsequent covert operations to overthrow Castro, born August 13, 1926, failed and he went on to become one of the world's longest-ruling heads of state. Fulgencio Batista died in Spain at age 72 on August 6, 1973. In late July 2006, an unwell Fidel Castro temporarily ceded power to his younger brother Raul. Fidel Castro officially stepped down in February 2008. ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ |
General Interest 1959 : Batista forced out by Castro-led revolution http://www.history.com/tdih.do?action=tdihVideoCategory&id=52297 45 BC: New Year's Day http://www.history.com/tdih.do?action=tdihArticleCategory&id=6763 1803 : Haitian independence proclaimed http://www.history.com/tdih.do?action=tdihArticleCategory&id=4632 1863 : Lincoln issues Emancipation Proclamation http://www.history.com/tdih.do?action=tdihArticleCategory&id=4633 1876 : First modern Mummers' Parade http://www.history.com/tdih.do?action=tdihArticleCategory&id=4634 |
Could the U.S. dollar be worth 40 percent less by next Chritmas?
By Jim Kunstler (author of the book the future after peak oil, The Long Emergency)
A summary of his commentary:
remnants of the middle class find it impossible to get car loans, or pay for fuel, or insurance, and that will set in motion a very impressive politics-of-grievance setting apart those who are still able to enjoy motoring and those who have been foreclosed from it.
other means will materialize.
of the kind that Americans are in the habit of enjoying.
A lot of families will lose everything. They will sift and disperse into the housing
owned by other family members -- parents, siblings -- and a strange new not-altogether comfortable kind of togetherness will become common. Over time, a lot of people will go looking for casual work "under-the-table"( and probably low-paying). To some degree, these workers will begin to look and act like a new servant class, and before too long they may be absorbed into the households of people who employ them. There will be plenty of room for them there.
2009 may be the point where we begin to understand what kinds of places will be more hospitable to human society further ahead. I maintain that our giant urban metroplexes have way overshot their sustainable scale and will contract severely. With all the economic hardship, we ought to expect a lot of demographic churning, people leaving hopeless places and moving on to something more promising. I believe we will see them move to smaller towns and smaller cities. The reorganization of the rural landscape into smaller-scaled farms has not begun to occur -- though 2009 might be very hard on
agribusiness, given the shortage of capital and if oil begins to march up in price by late winter. Eventually, the rural landscape will require the labor of many more people than is currently the case. Whatever else happens, 2009 will surely see a massive return to home
gardening as budgets become strained to the extreme.
Mexico's super-giant Cantarell oil field, the second-largest ever discovered after Saudi
Arabia's Ghawar field, has shown a 30 percent depletion rate in the past year alone.
Mexico is America's third largest source of imports My prediction for 2009 is that we will see two things occur, possibly at the same time: a resumption of rising prices, and spot shortages.
around the world, but they are still susceptible to vagaries in the oil markets and Black Swan events. As the U.S. consumer economy falls into a coma, and the shipping containers from China to WalMart get sparser, the Chinese government will face the wrath of millions of unemployed workers. I believe they will struggle through 2009, perhaps growing more surly as the U.S. dollar inflates and their holdings of Treasury bills begins to look more like a swindle.
Russia may be suffering economically for the moment due to the crash of oil prices, but they are energy resource-rich -- at least for the next couple of decades -- and if they don't like the current price, they can keep more of their oil in the ground until the price looks
more attractive.
NOTE: Also see the current issue of Foreign Affairs magazine, "The Great Crash, 2008," subtitle, A geopolitical setback for the West, by Roger C. Altman.
The over-arching geopolitical theme of 2009 will be the end of robust globalism as we've known it for some time. Reduced trade, competition for energy resources, sore feelings over debts and currencies will drive the nations inward or, at least, direct their energies toward their own regions.
will become clear that the Long Emergency is underway.
· the second on the 81st floor of the South Tower.
support more weight. The entire floor was then filled with server-
size Uninterrupted Power Supply (UPS) batteries.
These units were bolted to a raised floor about 3 feet above the
reinforced 81st floor. "The whole floor was batteries," he
said, "huge battery-looking things." They were "all black"
and "solid, very heavy" things that had been brought in during the
night. They had been put in place during the summer prior to 9/11,
he said.
data center," a secure computer room along the north and east sides
of the tower. And that's exactly where the plane hit the north wall
of the 95th floor.
NIST researchers were unable or unwilling to provide any description
of the contents of these crucial floors four years after 9/11.
http://www.rense.com/general75/thrm.htm
Examination of the forensic metallurgy of WTC steel “reveal a phenomenon never before observed in building fires: eutectic reactions, which caused ‘intergranular melting capable of turning a solid steel girder into Swiss cheese.’”
| The simple facts of temperatures:
Diffuse flames burn far cooler. Oxygen-starved diffuse flames are cooler yet. The fires in the towers were diffuse -- well below 800ºC. Their dark smoke showed they were oxygen-starved -- particularly in the South Tower. |
| Steel doesn't begin to melt until it reaches temperatures of between 1500 and 2000C, and it does not literally flow like lava until it gets towards 3000C, much hotter than the temperature jet fuel, or in the case of building seven, office material fires burn at. a far more extensive fire occurred in WTC 1 itself, prior to enhanced fireproofing of the building, on February 13, 1975. The fire burned at much higher temperatures for three hours and spread over six floors, including 65% of the 11th floor and the building core, yet it caused no significant damage to the steel structure and no trusses had to be replaced. |
November 22, 2004
In an effort to better understand the conditions that led to complete collapses of the World Trade Center Towers and WTC 7, we apply scanning-electron-microscope (SEM) and energy dispersive x-ray spectroscopy (XEDS) methods to analyze the dust generated, with an emphasis on observed micro-spheres in the WTC dust.
The formation of molten spheres with high iron contents along with other species in the
WTC dust required extremely high temperatures. Our results are compared with those of other laboratories. The temperatures required for the molten sphere-formation and evaporation of materials as observed in the WTC dust are significantly higher than temperatures associated with the burning of jet fuel and office materials in the WTC buildings.
The temperatures required for the observed spherule-formation and evaporation of materials observed in the WTC dust (table 1) are significantly higher than temperatures reachable by the burning of jet fuel and office materials in the WTC buildings (table 2). The temperatures required to melt iron (1,538 °C) and molybdenum (2,623 °C), and to vaporize lead (1,740 °C) and aluminosilicates (~2,760°C), are completely out of reach of the fires in the WTC buildings (maximum 1,100 °C). We wish to call attention to this discrepancy: the official
view implicating fires as the main cause for the ultimate collapses of the WTC Towers and WTC 7 (FEMA [13], NIST [15] ) is inadequate to explain this temperature gap and is therefore incomplete at best.
The formation of numerous metal-rich spherules is also remarkable, for it implies formation of high-temperature droplets of the molten metals, dispersed in the air where they cool to form spherules. We observe spherules with high iron and aluminum contents, a chemical signature which is not consistent with formation from melted steel.
The data provide strong evidence that chemical reactions which were both violent and highly-exothermic contributed to the destruction of the WTC buildings. NIST neglected the high-temperature and fragmentation evidence presented here: it appears nowhere in their final report [15]. Proposed new building codes based on the WTC disaster must address all available evidence for what caused the complete and rapid destruction of these skyscrapers. Understanding the mechanisms that led to the destruction of the World Trade Center will enable scientists and engineers to provide a safer environment for people using similar buildings and benefit firefighters who risk their lives trying to save others. Thus, a thorough investigation which considers these data, showing extremely high temperatures and severe fragmentation in the formation of small metal-rich spheres
during the WTC Towers destruction, is highly motivated. In particular, the repeatedly-delayed report on the destruction of WTC 7 on 9/11/2001 [21] should address these striking facts.
The extensive evidence that explosives were used at the WTC includes witness testimony (MacQueen 2006), overwhelming physical evidence (Griffin 2005, Hoffman et al 2005, Jones and Legge et al 2008) and simple common sense (Legge 2007). There is also substantial evidence that aluminothermic (thermite) materials were present at the WTC
(Jones 2007), and the presence of such materials can explain the existence of intense fire where it would not otherwise have existed. Additionally, despite agreement from all parties that the assumed availability of fuel allowed for the fires in any given location of each of the WTC buildings to last only twenty minutes (NIST 2007), the fires lasted much longer and produced extreme temperatures (Jones and Farrer et al 2008).
It turns out that explosive, sol-gel nano-thermites were developed by US government scientists, at Lawrence Livermore National Laboratories (LLNL) (Tillitson et al 1998, Gash et al 2000, Gash et al 2002). These LLNL scientists reported that --
"The sol-gel process is very amenable to dip-, spin-, and spray-coating technologies to coat surfaces. We have utilized this property to dip-coat various substrates to make sol-gel Fe,O,/ Al / Viton coatings. The energetic coating dries to give a nice adherent film. Preliminary experiments indicate that films of the hybrid material are self-propagating when ignited by thermal stimulus"
(Gash et al 2002).
The amazing correlation between floors of impact and floors of apparent failure suggests hat spray-on nano-thermite materials may have been applied to the steel components of the WTC buildings, underneath the upgraded fireproofing (Ryan 2008). This could have been done in such a way that very few people knew what was happening. The Port Authority’s engineering consultant Buro Happold, helping with evaluation of the
fireproofing upgrades, suggested the use of "alternative materials" (NIST 2005). Such alternative materials could have been spray-on nano-thermites substituted for intumescent paint or Interchar-like fireproofing primers (NASA 2006). It seems quite possible that this kind of substitution could have been made with few people noticing.
http://journalof911studies.com/volume/2008/Ryan_NIST_and_Nano-1.pdf
Major Protest Against Gaza Assault in Washington, D.C. - Friday, Jan. 2 at 3:30 pm
 |
Please post this event on your Facebook and MySpace pages,
and forward it widely to your friends and family.
Major Protest Against Gaza Assault
Rally & March in Washington, D.C.
Friday, January 2 at 3:30 pm
Rally at the Israeli Embassy - 3514 International Dr, NW
(Van Ness metro on the Red line; near Connecticut & Van Ness)
Followed by march ending at the Egyptian Embassy
3521 International Ct, NW
 |
| Yesterday, over 5,000 protesters in Washington, D.C. marched from the State Department to the White House. |
Yesterday, tens of thousands of people took to the streets in scores of cities across the country and around the world demanding an immediate end to the bombing of Gaza. In Washington D.C., 5,000 people gathered at the State Department and marched to the White House. Those taking to the streets included many children, teenagers and young adults. As the nighttime march entered the White House grounds, it took over and filled all of Pennsylvania Avenue with young people raising Palestinian flags at the White House fence. For a report on other demonstrations that happened all across the country, please visit the ANSWER Coalition website (see below).
The massacre of the Palestinian people continues. Nearly 400 Palestinians have been murdered. Israel has rejected any ceasefire, and the Egyptian government continues to block Palestinians who are trying to cross the border to escape the massive bombing campaign.
The ANSWER Coalition, Muslim American Society Freedom and the National Council of Arab Americans are calling for all progressive people to come out for a large-scale demonstration at the Israeli Embassy on Friday, January 2 at 3:30 pm. We will then march to the Egyptian Embassy.
Please make an urgently needed donation today.
Please make an urgently needed donation. We need your support to continue this work. Click here to donate online, where you can also find information on how to contribute by check.
Become a volunteer.
Our work is carried out entirely by volunteers. If you would like to become a volunteer, email info@answercoalition.org.
Read a report on demonstrations that were held all across the country.
To read a full report on the many demonstrations that were held in scores of cities across the country yesterday, Dec. 30, visit www.answercoalition.org.
A.N.S.W.E.R. Coalition
http://www.answercoalition.org/
info@internationalanswer.org
National Office in Washington DC: 202-544-3389
New York City: 212-694-8720
Los Angeles: 323-464-1636
San Francisco: 415-821-6545
Chicago: 773-463-0311
Seattle: 206-568-1661

"Resist Imperialism"
t-shirts
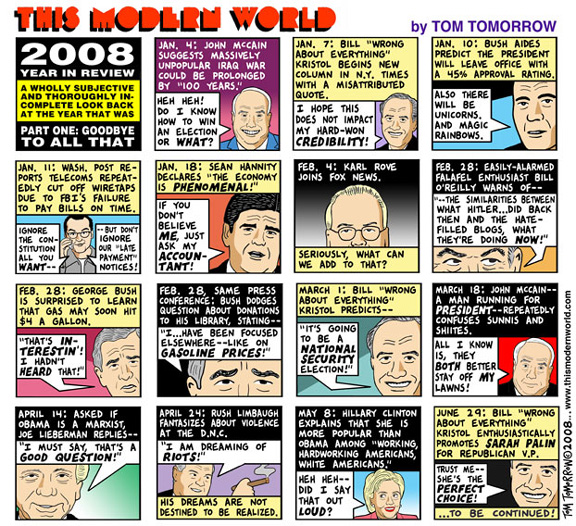
2008 Year in Review, Part One
More on Huffington Post...
Amb. Marc Ginsberg: Time for Hamas' Leaders to Go

AP/Khalil Hamra
Amb. Marc Ginsberg: Time for Hamas' Leaders to Go
Amb. Marc Ginsberg: In order order to understand what this struggle is all about, one must understand Hamas' goals, largely derived from its ideological paternity to the Egyptian Muslim Brothehood. As a Sunni extremist offshoot of the Brotherhood, Hamas' raison d'etre is Israel's destruction -- nothing less will do. Despite my instinctive belief that one should try to negotiate a way out of this dilemma no matter the odds, I have concluded that the only way out of this mess is to separate Hamas' entire military and political leadership from the oppressed citizenry of Gaza (and yes, it is absolutely a mischaracterization of fact to assert that Hamas is the legitimate ruler of Gaza). Easier said than done you say. But as long as Hamas rules Gaza, no amount of cajoling is going to end the vicious cycle of terror that Hamas is inflicting first and foremost on its own beaten-down Palestinian victims as well as on Israel.what is Click here to read more.
Mid-Tunnel Glimmerings
Tuesday 30 December 2008
by: Jean-Jacques Roth, Le Temps
Jean-Jacques Roth sees two glimmers of light in our current situation: the possibility of concerted global action on the economy and the overthrow of greed as an ultimate value. (Photo: Simon / Santacruzin)
What a year! Wherever one turns: vertigo. Financial massacre, destruction of value unprecedented in human history, the unanticipated become an everyday occurrence, the most learned predictions trampled by phenomena deemed unthinkable just twelve months ago ...
If the essential purpose of crises is to incite us to think from scratch, this one should, by virtue of its scale, soon deliver a totally new world.
But for the moment, it's the absence of visibility that strikes the mind. A brutal, but short, fever, or durable chaos, soon to be matched by more serious disorders? We are en route to the unknown. No alternative is ready to replace the foundations of capitalism. No theory has appeared to clothe the disillusions provoked by the all-powerful market's turmoil with Utopias.
That's not bad news. In the past, too many dramas, too many wars accompanied economic spasms and the revanchist egotism of their nationalist managements or the adventurism of their revolutionary consequences.
The most precious gain of our era is therefore not only the progress in economic science since the 1929 crisis. It is in the awareness of a global world and in the presence of the supranational constructions that awareness begot. Whether the European Union or the World Trade Organization, these institutions have in common the action of checking the return of everyman for himself - the outcome of which is stubbornly tragic. Let us imagine for a moment what the present crisis would be like were the Euro and tariff regulations not there to prevent the competitive devaluations and customs protections that the past gratified us with!
But today we have a higher threshold to cross. The need for global governance to remediate the effects of global warming is already apparent. Today, it's the economy that must be reformed. The emergency is so striking that it is shaking up the inertia of the balance of power frozen in place by the victors of the Second World War. Through this unsuspected constraint, the rebalancing of the multipolar world could finally receive a decisive push on the accelerator.
That would be the first revolution of the twenty-first century, but it is not in itself a guarantee of effectiveness. At the WTO, we see the exhausting work a compromise between a multitude of more equal partners requires.
Will the regulation of the financial planet encounter the same roadblocks? That must be feared, should the return of politics limit itself to the invocation of having other people pay: the neighbors, future generations, but certainly not the yield on my government pension! The real requirement of the political is also to promote acceptance of the individual discipline and sacrifices that collective transformations demand.
It is obviously more convenient to wait for the bearer of hope - Barack Obama, of course. Will he be able to transform hope into acts and to become the president of a more reasonable world? His faultless career so far allows us to continue to believe it.
There's another reason to be gladdened in the short term. For this debacle will have at least defeated one fraud: that of a cupidity that had become insane, erecting its obscene fatuousness as a universal totem. We'll never be altogether done with it, of course, but will do at least two retakes before making it a cardinal value. In the tunnel we have entered, that's a glimmer at least as strong as the promises of concerted action on our common destiny.
So let us bet on the light: Happy New Year!
--------
Translation: Truthout French language editor Leslie Thatcher.
Drug Companies and Doctors: A Story of Corruption
Monday 15 December 2008
by: Marcia Angell, The New York Review of Books
The suppression of unfavorable pharmaceutical research in medical journals is the subject of Alison Bass's book, "Side Effects: A Prosecutor, a Whistleblower, and a Bestselling Antidepressant on Trial." (Photo: Pat Greenhouse / The Boston Globe)
Side Effects: A Prosecutor, a Whistleblower, and a Bestselling Antidepressant on Trial
by Alison Bass
Algonquin Books of Chapel Hill, 260 pp.
Our Daily Meds: How the Pharmaceutical Companies Transformed Themselves into Slick Marketing Machines and Hooked the Nation on Prescription Drugs
by Melody Petersen
Sarah Crichton/Farrar, Straus and Giroux, 432 pp.
Shyness: How Normal Behavior Became a Sickness
by Christopher Lane
Yale University Press, 263 pp.
Recently Senator Charles Grassley, ranking Republican on the Senate Finance Committee, has been looking into financial ties between the pharmaceutical industry and the academic physicians who largely determine the market value of prescription drugs. He hasn't had to look very hard.
Take the case of Dr. Joseph L. Biederman, professor of psychiatry at Harvard Medical School and chief of pediatric psychopharmacology at Harvard's Massachusetts General Hospital. Thanks largely to him, children as young as two years old are now being diagnosed with bipolar disorder and treated with a cocktail of powerful drugs, many of which were not approved by the Food and Drug Administration (FDA) for that purpose and none of which were approved for children below ten years of age.
Legally, physicians may use drugs that have already been approved for a particular purpose for any other purpose they choose, but such use should be based on good published scientific evidence. That seems not to be the case here. Biederman's own studies of the drugs he advocates to treat childhood bipolar disorder were, as The New York Times summarized the opinions of its expert sources, "so small and loosely designed that they were largely inconclusive."(1)
In June, Senator Grassley revealed that drug companies, including those that make drugs he advocates for childhood bipolar disorder, had paid Biederman $1.6 million in consulting and speaking fees between 2000 and 2007. Two of his colleagues received similar amounts. After the revelation, the president of the Massachusetts General Hospital and the chairman of its physician organization sent a letter to the hospital's physicians expressing not shock over the enormity of the conflicts of interest, but sympathy for the beneficiaries: "We know this is an incredibly painful time for these doctors and their families, and our hearts go out to them."
Or consider Dr. Alan F. Schatzberg, chair of Stanford's psychiatry department and president-elect of the American Psychiatric Association. Senator Grassley found that Schatzberg controlled more than $6 million worth of stock in Corcept Therapeutics, a company he cofounded that is testing mifepristone - the abortion drug otherwise known as RU-486 - as a treatment for psychotic depression. At the same time, Schatzberg was the principal investigator on a National Institute of Mental Health grant that included research on mifepristone for this use and he was coauthor of three papers on the subject. In a statement released in late June, Stanford professed to see nothing amiss in this arrangement, although a month later, the university's counsel announced that it was temporarily replacing Schatzberg as principal investigator "to eliminate any misunderstanding."
Perhaps the most egregious case exposed so far by Senator Grassley is that of Dr. Charles B. Nemeroff, chair of Emory University's department of psychiatry and, along with Schatzberg, coeditor of the influential Textbook of Psychopharmacology.(2) Nemeroff was the principal investigator on a five-year $3.95 million National Institute of Mental Health grant - of which $1.35 million went to Emory for overhead - to study several drugs made by GlaxoSmithKline. To comply with university and government regulations, he was required to disclose to Emory income from GlaxoSmithKline, and Emory was required to report amounts over $10,000 per year to the National Institutes of Health, along with assurances that the conflict of interest would be managed or eliminated.
But according to Senator Grassley, who compared Emory's records with those from the company, Nemeroff failed to disclose approximately $500,000 he received from GlaxoSmithKline for giving dozens of talks promoting the company's drugs. In June 2004, a year into the grant, Emory conducted its own investigation of Nemeroff's activities, and found multiple violations of its policies. Nemeroff responded by assuring Emory in a memorandum, "In view of the NIMH/Emory/GSK grant, I shall limit my consulting to GSK to under $10,000/year and I have informed GSK of this policy." Yet that same year, he received $171,031 from the company, while he reported to Emory just $9,999 - a dollar shy of the $10,000 threshold for reporting to the National Institutes of Health.
Emory benefited from Nemeroff's grants and other activities, and that raises the question of whether its lax oversight was influenced by its own conflicts of interest. As reported by Gardiner Harris in TheNew York Times,(3) Nemeroff himself had pointed out his value to Emory in a 2000 letter to the dean of the medical school, in which he justified his membership on a dozen corporate advisory boards by saying:
Surely you remember that Smith-Kline Beecham Pharmaceuticals donated an endowed chair to the department and there is some reasonable likelihood that Janssen Pharmaceuticals will do so as well. In addition, Wyeth-Ayerst Pharmaceuticals has funded a Research Career Development Award program in the department, and I have asked both AstraZeneca Pharmaceuticals and Bristol-Meyers [sic] Squibb to do the same. Part of the rationale for their funding our faculty in such a manner would be my service on these boards.
Because these psychiatrists were singled out by Senator Grassley, they received a great deal of attention in the press, but similar conflicts of interest pervade medicine. (The senator is now turning his attention to cardiologists.) Indeed, most doctors take money or gifts from drug companies in one way or another. Many are paid consultants, speakers at company-sponsored meetings, ghost-authors of papers written by drug companies or their agents,(4) and ostensible "researchers" whose contribution often consists merely of putting their patients on a drug and transmitting some token information to the company. Still more doctors are recipients of free meals and other out-and-out gifts. In addition, drug companies subsidize most meetings of professional organizations and most of the continuing medical education needed by doctors to maintain their state licenses.
No one knows the total amount provided by drug companies to physicians, but I estimate from the annual reports of the top nine US drug companies that it comes to tens of billions of dollars a year. By such means, the pharmaceutical industry has gained enormous control over how doctors evaluate and use its own products. Its extensive ties to physicians, particularly senior faculty at prestigious medical schools, affect the results of research, the way medicine is practiced, and even the definition of what constitutes a disease.
Consider the clinical trials by which drugs are tested in human subjects.(5) Before a new drug can enter the market, its manufacturer must sponsor clinical trials to show the Food and Drug Administration that the drug is safe and effective, usually as compared with a placebo or dummy pill. The results of all the trials (there may be many) are submitted to the FDA, and if one or two trials are positive - that is, they show effectiveness without serious risk - the drug is usually approved, even if all the other trials are negative. Drugs are approved only for a specified use - for example, to treat lung cancer - and it is illegal for companies to promote them for any other use.
But physicians may prescribe approved drugs "off label" - i.e., without regard to the specified use - and perhaps as many as half of all prescriptions are written for off-label purposes. After drugs are on the market, companies continue to sponsor clinical trials, sometimes to get FDA approval for additional uses, sometimes to demonstrate an advantage over competitors, and often just as an excuse to get physicians to prescribe such drugs for patients. (Such trials are aptly called "seeding" studies.)
Since drug companies don't have direct access to human subjects, they need to outsource their clinical trials to medical schools, where researchers use patients from teaching hospitals and clinics, or to private research companies (CROs), which organize office-based physicians to enroll their patients. Although CROs are usually faster, sponsors often prefer using medical schools, in part because the research is taken more seriously, but mainly because it gives them access to highly influential faculty physicians - referred to by the industry as "thought-leaders" or "key opinion leaders" (KOLs). These are the people who write textbooks and medical journal papers, issue practice guidelines (treatment recommendations), sit on FDA and other governmental advisory panels, head professional societies, and speak at the innumerable meetings and dinners that take place every year to teach clinicians about prescription drugs. Having KOLs like Dr. Biederman on the payroll is worth every penny spent.
A few decades ago, medical schools did not have extensive financial dealings with industry, and faculty investigators who carried out industry-sponsored research generally did not have other ties to their sponsors. But schools now have their own manifold deals with industry and are hardly in a moral position to object to their faculty behaving in the same way. A recent survey found that about two thirds of academic medical centers hold equity interest in companies that sponsor research within the same institution.(6) A study of medical school department chairs found that two thirds received departmental income from drug companies and three fifths received personal income.(7) In the 1980s medical schools began to issue guidelines governing faculty conflicts of interest but they are highly variable, generally quite permissive, and loosely enforced.
Because drug companies insist as a condition of providing funding that they be intimately involved in all aspects of the research they sponsor, they can easily introduce bias in order to make their drugs look better and safer than they are. Before the 1980s, they generally gave faculty investigators total responsibility for the conduct of the work, but now company employees or their agents often design the studies, perform the analysis, write the papers, and decide whether and in what form to publish the results. Sometimes the medical faculty who serve as investigators are little more than hired hands, supplying patients and collecting data according to instructions from the company.
In view of this control and the conflicts of interest that permeate the enterprise, it is not surprising that industry-sponsored trials published in medical journals consistently favor sponsors' drugs - largely because negative results are not published, positive results are repeatedly published in slightly different forms, and a positive spin is put on even negative results. A review of seventy-four clinical trials of antidepressants, for example, found that thirty-seven of thirty-eight positive studies were published.(8) But of the thirty-six negative studies, thirty-three were either not published or published in a form that conveyed a positive outcome. It is not unusual for a published paper to shift the focus from the drug's intended effect to a secondary effect that seems more favorable.
The suppression of unfavorable research is the subject of Alison Bass's engrossing book, Side Effects: A Prosecutor, a Whistleblower, and a Bestselling Antidepressant on Trial. This is the story of how the British drug giant GlaxoSmithKline buried evidence that its top-selling antidepressant, Paxil, was ineffective and possibly harmful to children and adolescents. Bass, formerly a reporter for the Boston Globe, describes the involvement of three people - a skeptical academic psychiatrist, a morally outraged assistant administrator in Brown University's department of psychiatry (whose chairman received in 1998 over $500,000 in consulting fees from drug companies, including GlaxoSmithKline), and an indefatigable New York assistant attorney general. They took on GlaxoSmithKline and part of the psychiatry establishment and eventually prevailed against the odds.
The book follows the individual struggles of these three people over many years, culminating with GlaxoSmithKline finally agreeing in 2004 to settle charges of consumer fraud for $2.5 million (a tiny fraction of the more than $2.7 billion in yearly Paxil sales about that time). It also promised to release summaries of all clinical trials completed after December 27, 2000. Of much greater significance was the attention called to the deliberate, systematic practice of suppressing unfavorable research results, which would never have been revealed without the legal discovery process. Previously undisclosed, one of GlaxoSmithKline's internal documents said, "It would be commercially unacceptable to include a statement that efficacy had not been demonstrated, as this would undermine the profile of paroxetine [Paxil]."(9)
Many drugs that are assumed to be effective are probably little better than placebos, but there is no way to know because negative results are hidden. One clue was provided six years ago by four researchers who, using the Freedom of Information Act, obtained FDA reviews of every placebo-controlled clinical trial submitted for initial approval of the six most widely used antidepressant drugs approved between 1987 and 1999 - Prozac, Paxil, Zoloft, Celexa, Serzone, and Effexor.(10) They found that on average, placebos were 80 percent as effective as the drugs. The difference between drug and placebo was so small that it was unlikely to be of any clinical significance. The results were much the same for all six drugs: all were equally ineffective. But because favorable results were published and unfavorable results buried (in this case, within the FDA), the public and the medical profession believed these drugs were potent antidepressants.
Clinical trials are also biased through designs for research that are chosen to yield favorable results for sponsors. For example, the sponsor's drug may be compared with another drug administered at a dose so low that the sponsor's drug looks more powerful. Or a drug that is likely to be used by older people will be tested in young people, so that side effects are less likely to emerge. A common form of bias stems from the standard practice of comparing a new drug with a placebo, when the relevant question is how it compares with an existing drug. In short, it is often possible to make clinical trials come out pretty much any way you want, which is why it's so important that investigators be truly disinterested in the outcome of their work.
Conflicts of interest affect more than research. They also directly shape the way medicine is practiced, through their influence on practice guidelines issued by professional and governmental bodies, and through their effects on FDA decisions. A few examples: in a survey of two hundred expert panels that issued practice guidelines, one third of the panel members acknowledged that they had some financial interest in the drugs they considered.(11) In 2004, after the National Cholesterol Education Program called for sharply lowering the desired levels of "bad" cholesterol, it was revealed that eight of nine members of the panel writing the recommendations had financial ties to the makers of cholesterol-lowering drugs.(12) Of the 170 contributors to the most recent edition of the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders (DSM), ninety-five had financial ties to drug companies, including all of the contributors to the sections on mood disorders and schizophrenia.(13) Perhaps most important, many members of the standing committees of experts that advise the FDA on drug approvals also have financial ties to the pharmaceutical industry.(14)
In recent years, drug companies have perfected a new and highly effective method to expand their markets. Instead of promoting drugs to treat diseases, they have begun to promote diseases to fit their drugs. The strategy is to convince as many people as possible (along with their doctors, of course) that they have medical conditions that require long-term drug treatment. Sometimes called "disease-mongering," this is a focus of two new books: Melody Petersen's Our Daily Meds: How the Pharmaceutical Companies Transformed Themselves into Slick Marketing Machines and Hooked the Nation on Prescription Drugs and Christopher Lane's Shyness: How Normal Behavior Became a Sickness.
To promote new or exaggerated conditions, companies give them serious-sounding names along with abbreviations. Thus, heartburn is now "gastro-esophageal reflux disease" or GERD; impotence is "erectile dysfunction" or ED; premenstrual tension is "premenstrual dysphoric disorder" or PMMD; and shyness is "social anxiety disorder" (no abbreviation yet). Note that these are ill-defined chronic conditions that affect essentially normal people, so the market is huge and easily expanded. For example, a senior marketing executive advised sales representatives on how to expand the use of Neurontin: "Neurontin for pain, Neurontin for monotherapy, Neurontin for bipolar, Neurontin for everything."(15) It seems that the strategy of the drug marketers - and it has been remarkably successful - is to convince Americans that there are only two kinds of people: those with medical conditions that require drug treatment and those who don't know it yet. While the strategy originated in the industry, it could not be implemented without the complicity of the medical profession.
Melody Petersen, who was a reporter for The New York Times, has written a broad, convincing indictment of the pharmaceutical industry.(16) She lays out in detail the many ways, both legal and illegal, that drug companies can create "blockbusters" (drugs with yearly sales of over a billion dollars) and the essential role that KOLs play. Her main example is Neurontin, which was initially approved only for a very narrow use - to treat epilepsy when other drugs failed to control seizures. By paying academic experts to put their names on articles extolling Neurontin for other uses - bipolar disease, post-traumatic stress disorder, insomnia, restless legs syndrome, hot flashes, migraines, tension headaches, and more - and by funding conferences at which these uses were promoted, the manufacturer was able to parlay the drug into a blockbuster, with sales of $2.7 billion in 2003. The following year, in a case covered extensively by Petersen for the Times, Pfizer pleaded guilty to illegal marketing and agreed to pay $430 million to resolve the criminal and civil charges against it. A lot of money, but for Pfizer, it was just the cost of doing business, and well worth it because Neurontin continued to be used like an all-purpose tonic, generating billions of dollars in annual sales.
Christopher Lane's book has a narrower focus - the rapid increase in the number of psychiatric diagnoses in the American population and in the use of psychoactive drugs (drugs that affect mental states) to treat them. Since there are no objective tests for mental illness and the boundaries between normal and abnormal are often uncertain, psychiatry is a particularly fertile field for creating new diagnoses or broadening old ones.(17) Diagnostic criteria are pretty much the exclusive province of the current edition of the Diagnostic and Statistical Manual of Mental Disorders, which is the product of a panel of psychiatrists, most of whom, as I mentioned earlier, had financial ties to the pharmaceutical industry. Lane, a research professor of literature at Northwestern University, traces the evolution of the DSM from its modest beginnings in 1952 as a small, spiral-bound handbook (DSM-I) to its current 943-page incarnation (the revised version of DSM-IV) as the undisputed "bible" of psychiatry - the standard reference for courts, prisons, schools, insurance companies, emergency rooms, doctors' offices, and medical facilities of all kinds.
Given its importance, you might think that the DSM represents the authoritative distillation of a large body of scientific evidence. But Lane, using unpublished records from the archives of the American Psychiatric Association and interviews with the princi-pals, shows that it is instead the product of a complex of academic politics, personal ambition, ideology, and, perhaps most important, the influence of the pharmaceutical industry. What the DSM lacks is evidence. Lane quotes one contributor to the DSM-III task force:
There was very little systematic research, and much of the research that existed was really a hodgepodge - scattered, inconsistent, and ambiguous. I think the majority of us recognized that the amount of good, solid science upon which we were making our decisions was pretty modest.
Lane uses shyness as his case study of disease-mongering in psychiatry. Shyness as a psychiatric illness made its debut as "social phobia" in DSM-III in 1980, but was said to be rare. By 1994, when DSM-IV was published, it had become "social anxiety disorder," now said to be extremely common. According to Lane, GlaxoSmithKline, hoping to boost sales for its antidepressant, Paxil, decided to promote social anxiety disorder as "a severe medical condition." In 1999, the company received FDA approval to market the drug for social anxiety disorder. It launched an extensive media campaign to do it, including posters in bus shelters across the country showing forlorn individuals and the words "Imagine being allergic to people...," and sales soared. Barry Brand, Paxil's product director, was quoted as saying, "Every marketer's dream is to find an unidentified or unknown market and develop it. That's what we were able to do with social anxiety disorder."
Some of the biggest blockbusters are psychoactive drugs. The theory that psychiatric conditions stem from a biochemical imbalance is used as a justification for their widespread use, even though the theory has yet to be proved. Children are particularly vulnerable targets. What parents dare say "No" when a physician says their difficult child is sick and recommends drug treatment? We are now in the midst of an apparent epidemic of bipolar disease in children (which seems to be replacing attention-deficit hyperactivity disorder as the most publicized condition in childhood), with a forty-fold increase in the diagnosis between 1994 and 2003.(18) These children are often treated with multiple drugs off-label, many of which, whatever their other properties, are sedating, and nearly all of which have potentially serious side effects.
The problems I've discussed are not limited to psychiatry, although they reach their most florid form there. Similar conflicts of interest and biases exist in virtually every field of medicine, particularly those that rely heavily on drugs or devices. It is simply no longer possible to believe much of the clinical research that is published, or to rely on the judgment of trusted physicians or authoritative medical guidelines. I take no pleasure in this conclusion, which I reached slowly and reluctantly over my two decades as an editor of TheNew England Journal of Medicine.
One result of the pervasive bias is that physicians learn to practice a very drug-intensive style of medicine. Even when changes in lifestyle would be more effective, doctors and their patients often believe that for every ailment and discontent there is a drug. Physicians are also led to believe that the newest, most expensive brand-name drugs are superior to older drugs or generics, even though there is seldom any evidence to that effect because sponsors do not usually compare their drugs with older drugs at equivalent doses. In addition, physicians, swayed by prestigious medical school faculty, learn to prescribe drugs for off-label uses without good evidence of effectiveness.
It is easy to fault drug companies for this situation, and they certainly deserve a great deal of blame. Most of the big drug companies have settled charges of fraud, off-label marketing, and other offenses. TAP Pharmaceuticals, for example, in 2001 pleaded guilty and agreed to pay $875 million to settle criminal and civil charges brought under the federal False Claims Act over its fraudulent marketing of Lupron, a drug used for treatment of prostate cancer. In addition to GlaxoSmithKline, Pfizer, and TAP, other companies that have settled charges of fraud include Merck, Eli Lilly, and Abbott. The costs, while enormous in some cases, are still dwarfed by the profits generated by these illegal activities, and are therefore not much of a deterrent. Still, apologists might argue that the pharmaceutical industry is merely trying to do its primary job - further the interests of its investors - and sometimes it goes a little too far.
Physicians, medical schools, and professional organizations have no such excuse, since their only fiduciary responsibility is to patients. The mission of medical schools and teaching hospitals - and what justifies their tax-exempt status - is to educate the next generation of physicians, carry out scientifically important research, and care for the sickest members of society. It is not to enter into lucrative commercial alliances with the pharmaceutical industry. As reprehensible as many industry practices are, I believe the behavior of much of the medical profession is even more culpable.(19) Drug companies are not charities; they expect something in return for the money they spend, and they evidently get it or they wouldn't keep paying.
So many reforms would be necessary to restore integrity to clinical research and medical practice that they cannot be summarized briefly. Many would involve congressional legislation and changes in the FDA, including its drug approval process. But there is clearly also a need for the medical profession to wean itself from industry money almost entirely. Although industry-academic collaboration can make important scientific contributions, it is usually in carrying out basic research, not clinical trials, and even here, it is arguable whether it necessitates the personal enrichment of investigators. Members of medical school faculties who conduct clinical trials should not accept any payments from drug companies except research support, and that support should have no strings attached, including control by drug companies over the design, interpretation, and publication of research results.
Medical schools and teaching hospitals should rigorously enforce that rule, and should not enter into deals with companies whose products members of their faculty are studying. Finally, there is seldom a legitimate reason for physicians to accept gifts from drug companies, even small ones, and they should pay for their own meetings and continuing education.
After much unfavorable publicity, medical schools and professional organizations are beginning to talk about controlling conflicts of interest, but so far the response has been tepid. They consistently refer to "potential" conflicts of interest, as though that were different from the real thing, and about disclosing and "managing" them, not about prohibiting them. In short, there seems to be a desire to eliminate the smell of corruption, while keeping the money. Breaking the dependence of the medical profession on the pharmaceutical industry will take more than appointing committees and other gestures. It will take a sharp break from an extremely lucrative pattern of behavior. But if the medical profession does not put an end to this corruption voluntarily, it will lose the confidence of the public, and the government (not just Senator Grassley) will step in and impose regulation. No one in medicine wants that.
Notes
(1)Gardiner Harris and Benedict Carey, "Researchers Fail to Reveal Full Drug Pay," The New York Times, June 8, 2008.
(2)Most of the information in these paragraphs, including Nemeroff's quote in the summer of 2004, is drawn from a long letter written by Senator Grassley to James W. Wagner, President of Emory University, on October 2, 2008.
(3)See Gardiner Harris, "Leading Psychiatrist Didn't Report Drug Makers' Pay," The New York Times, October 4, 2008.
(4)Senator Grassley is current investigating Wyeth for paying a medical writing firm to ghost-write articles favorable to its hormone-replacement drug Prempro.
(5)Some of this material is drawn from my article "Industry-Sponsored Clinical Research: A Broken System," TheJournal of the American Medical Association, September 3, 2008.
(6)Justin E. Bekelman et al., "Scope and Impact of Financial Conflicts of Interest in Biomedical Research: A Systematic Review," The Journal of the American Medical Association, January 22, 2003.
(7)Eric G. Campbell et al., "Institutional Academic-Industry Relationships," The Journal of the American Medical Association, October 17, 2007.
(8)Erick H. Turner et al., "Selective Publication of Antidepressant Trials and Its Influence on Apparent Efficacy," The New England Journal of Medicine, January 17, 2008.
(9)See Wayne Kondro and Barb Sibbald, "Drug Company Experts Advised Staff to Withhold Data About SSRI Use in Children," Canadian Medical Association Journal, March 2, 2004.
(10)Irving Kirsch et al., "The Emperor's New Drugs: An Analysis of Antidepressant Medication Data Submitted to the US Food and Drug Administration," Prevention & Treatment, July 15, 2002.
(11)Rosie Taylor and Jim Giles, "Cash Interests Taint Drug Advice," Nature, October 20, 2005.
(12)David Tuller, "Seeking a Fuller Picture of Statins," The New York Times, July 20, 2004.
(13)Lisa Cosgrove et al., "Financial Ties Between DSM-IV Panel Members and the Pharmaceutical Industry," Psychotherapy and Psychosomatics, Vol. 75, No. 3 (2006).
(14)On August 4, 2008, the FDA announced that $50,000 is now the "maximum personal financial interest an advisor may have in all companies that may be affected by a particular meeting." Waivers may be granted for amounts less than that.
(15)See Petersen, Our Daily Meds, p. 224.
(16)Petersen's book is a part of a second wave of books exposing the deceptive practices of the pharmaceutical industry. The first included Katharine Greider's The Big Fix: How the Pharmaceutical Industry Rips Off American Consumers (PublicAffairs, 2003), Merrill Goozner's The $800 Million Pill: The Truth Behind the Cost of New Drugs (University of California Press, 2004), Jerome Avorn's Powerful Medicines: The Benefits, Risks, and Costs of Prescription Drugs (Knopf, 2004), John Abramson's Overdo$ed America: The Broken Promise of American Medicine (HarperCollins, 2004), and my own The Truth About the Drug Companies: How They Deceive Us and What to Do About It (Random House, 2004).
(17)See the review by Frederick Crews of Lane's book and two others, The New York Review, December 6, 2007.
(18)See Gardiner Harris and Benedict Carey, "Researchers Fail to Reveal Full Drug Pay," The New York Times, June 8, 2008.
(19)This point is made powerfully in Jerome P. Kassirer's disturbing book, On the Take: How Medicine's Complicity With Big Business Can Endanger Your Health (Oxford University Press, 2005).